| |
|
 |
|
 |
|
|
Radon is a radioactive, colorless, odorless and tasteless inert gas which is recognized to be carcinogenic. It is a radioactive by-product from the radioactive decay of Uranium. It exists in most of the geological structures. It is one of the 92 self-generated elements and is not a pollutant produced by humans.
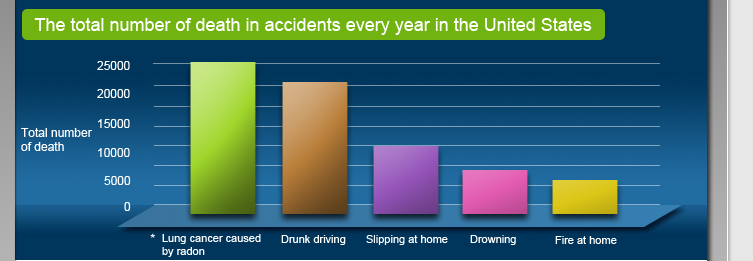
People have become aware of the harm of radon on human health since 1980s. When radon decays, it will release high-energy alpha particles which will seriously damage our lungs and cause lung cancer. According to the estimation of the U.S. Environmental Protection Agency, about 20,000 people die of excessive radon inhalation every year. This is more than the number of death caused by drunk driving*. |
|
 |
As radon is colorless and tasteless, only special instruments or equipment can measure its concentration. The unit of measurement of radon is pCi/l (picocuries per litre of air).
The average outdoor radon concentration in the United States is 0.4 pCi/L. The United States National Academy of Sciences has estimated that each year among the approximate 20,000 lung cancer cases which are caused by radon, 800 of them are due to inhalation of radon in outdoor areas. The higher the concentration of radon in the environment, the higher the chance of suffering from lung cancer. When the radon concentration is increased by 2.7 pCi/L, the chance of suffering from lung cancer will be increased by 16%.
If the ventilation of indoor environment is poor, radon will accumulate so the concentration must be higher than that in outdoor environment. Currently there is no “safety level” for radon in any country as the safest situation is no radon. However, this is impossible as radon will appear naturally. Therefore, most of the health organizations, including the U.S. Environmental Protection Agency, set 4 pCi/l as the “acceptable level” for radon. If the indoor radon concentration is higher than 4 pCi/l, then improvement projects are needed to protect health.
*Radon is one of the common indoor air pollutants listed by the Indoor Air Quality Information Centre. Homeasy also provides a comprehensive IAQ test. Welcome to contact us for more details. |
|
 |
Physical properties
Radon is a colorless and tasteless gas and thus cannot be detected by human senses. Under standard temperature and pressure, the density of an atomic radon is 9.73 kg/m3 while the atmospheric density is 1.217 kg/m3. Therefore, the density of radon is about 8 times of that of the atmosphere. Radon is the heaviest gas under room temperature.
Chemical properties
As an inert gas, radon has inactive chemical reactions. Radon has 8 outermost shell electrons, meaning that it has obtained an octet structure already. As radon is radioactive, it seldom appears in chemical experiments. It is hard for radon to react with other elements to form compounds. However, radon can react with strong fluorinating agents such as fluorine to become radon fluoride.
Isotopes
Radon has 34 known isotopes from radon-195 to radon-228. Among them, the most stable isotope is radon-222, which is a decay product of radium-226. Radon-222 will emitαparticles during decay, with a half-life of 3.823 days. Radon-220, also known as thoron, is a decay product of thorium. It will emitαparticles during decay, with a half-life of 55.6 seconds. Radon-219, also known as action, is a decay product of actinium. It will emitαparticles during decay, with a half-life of 3.96 seconds. |
|
|
| Scientific research has shown as children have higher breathing rate and faster cell division rate, radon is more harmful to children.
According to the U.S. Environmental Protection Agency, concentrated radon will cause adverse effects to body blood cells. The death rate of poisoning caused by radon is 100 times higher than that caused by carbon monoxide.
According to research by foreign countries, for home with poor ventilation, if it is built by stone materials which contains radon, then the amount of radiation inside the home will even be 2 times higher than the statutory safety dose for operators of nuclear power plants.
|
|
 |
Construction materials such as granite, brick sand, cement and gypsum are the main source of indoor radon. Natural stone materials containing radioactive elements will emit radon more easily.
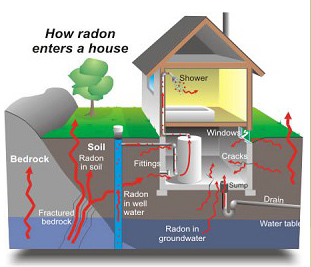
Radon can get into the basement, ground floor or flats on higher level of a building through the cracks and gaps on the wall, floor or on the ground. |
| |
| The main sources of indoor radon will be different for different types of buildings. |
| 1. |
For low-rise structures with less than 3 storeys (e.g. houses, villa): Rock (soil) under the base |
|
 |
|
| 2. |
For high-rise structures with more than 3 storeys: Indoor construction materials, especially granite or ceramic tiles |
|
 |
|
|
|
|
 |
|
|